Wednesday, December 8, 2010
Friday, December 3, 2010
Decemeber 3, 2010
Today we learned how to :
Calculate the Empirical Formula! (organic compounds)
REMINDER! QUIZ on Empirical Formula, Percentage Composition
-Empirical Formulas including organic compound
-burning the compound (react it with O2)
-collecting and weighing of the products resulting from the burning of the compound
-from the mass of the products, the moles of each element in the original organic compound can be calculated
Eg. What is the EF of a compound when 5.0g sample is burned producing 15.0g of CO2 and 8.18g of H20.
Mol CO2 = 15g x (1mol/44.0g) = 0.341 moles
Mol H2O = 8.18g x (1 mol/18.02g) = 0.454 moles
Mol C = 0.431 moles because there is only 1 C in ( CO2) = 0.341 moles | 1.0 | 3
| |
Mol H = O.454 moles x 2 because there is 2 H in (H2O) = 0.908 moles | 2.6 | 8
Mol H = O.454 moles x 2 because there is 2 H in (H2O) = 0.908 moles | 2.6 | 8
EF = C3H8
0.341 moles of C x ( 12.0g/1 mol ) = 4.092g of C
0.908 moles of H x ( 1.0g/ 1 mol ) = 0.908g of H
Total Mass = (C) + (H) should equal to 5
so if the final answer equals the amount given in the question, you can reassure that there are not O in the EF.
Another Perfect example and step by step procedures!
A good video explaining how to calculate the Empirical Formula
Thursday, December 2, 2010
December 1 2010 - Empirical and Molecular Formulas
Happy December ~ ! Only about 2 more weeks until it's winter break. (:
So we learned about the empirical formula and the molecular formula. What exactly are they?
The empirical formula is a formula used to shrink down the ration of atoms (or moles) in a formula. All ionic compounds are empirical formulas!
For example, C4H10, which is butane in its molecular formula, would be C2H5 in its empirical formula.
The molecular formula is the form of the empirical formula showing the actual number of atoms that combine to form a molecule.
To calculate the molecular formula, you use this equation :
n = molar mass of the compound / molar mass of the empirical formula
So we learned about the empirical formula and the molecular formula. What exactly are they?
The empirical formula is a formula used to shrink down the ration of atoms (or moles) in a formula. All ionic compounds are empirical formulas!
For example, C4H10, which is butane in its molecular formula, would be C2H5 in its empirical formula.
The molecular formula is the form of the empirical formula showing the actual number of atoms that combine to form a molecule.
To calculate the molecular formula, you use this equation :
n = molar mass of the compound / molar mass of the empirical formula
Tuesday, November 30, 2010
Nov 29 2010 - percent composition
Today we went over percent composition.
Percent composition of a compound shows that how much of each elements in the compound and which elements are in the compound.
Percentage compound = mass of element / mass of compound x 100 %
Ex 1. What is the percentage composition of CuCl2?
Total MM(Molar Mass) = 134.5g/mol
% of Cu = 63.5/134.5 x 100 % = 47.2 %
% of Cl = 71.0/134.5 x 100% = 52.8 %
2. What is the percentage ocomposition of CaO?
Total MM = 56.1g/mol
% of Ca = 40.1/56.1 x 100% = 71.5 %
% of O = 16.0/56.1 x 100 % = 29.5
3. Calculate percentage composition of the bolded components of Na2SO4.
Total MM = 142.1g/mol
MM of all the SO4 = 96.1g/mol
% of SO4 = 96.1/142.1 x 100 % = 67.6 %
4. Calculate percentage composition of NaCl.
Total MM = 58.5g/mol
% of Na = 23.0/58.5 x 100 % = 39.3 %
% of Cl = 35.5/58.5 x 100% = 60.7 %
5. Calculate the percentage of bolded components of (NH4)3PO4.
Total MM = 149.1
MM of all the NH4 = 54.0
% of NH4 = 54.0 / 149.1 x 100 % = 36.2 %
Percent composition of a compound shows that how much of each elements in the compound and which elements are in the compound.
Percentage compound = mass of element / mass of compound x 100 %
Ex 1. What is the percentage composition of CuCl2?
Total MM(Molar Mass) = 134.5g/mol
% of Cu = 63.5/134.5 x 100 % = 47.2 %
% of Cl = 71.0/134.5 x 100% = 52.8 %
2. What is the percentage ocomposition of CaO?
Total MM = 56.1g/mol
% of Ca = 40.1/56.1 x 100% = 71.5 %
% of O = 16.0/56.1 x 100 % = 29.5
3. Calculate percentage composition of the bolded components of Na2SO4.
Total MM = 142.1g/mol
MM of all the SO4 = 96.1g/mol
% of SO4 = 96.1/142.1 x 100 % = 67.6 %
4. Calculate percentage composition of NaCl.
Total MM = 58.5g/mol
% of Na = 23.0/58.5 x 100 % = 39.3 %
% of Cl = 35.5/58.5 x 100% = 60.7 %
5. Calculate the percentage of bolded components of (NH4)3PO4.
Total MM = 149.1
MM of all the NH4 = 54.0
% of NH4 = 54.0 / 149.1 x 100 % = 36.2 %
Tuesday, November 23, 2010
November 23, 2010
Today, we went more in detail the amazing wonders of Mole, and the conversion powers it possess.
-Not only are we able to calculate the number of particles, formula units, molecules, atoms from Avogadro's number
-We are now able to use the molar mass of any molecule to calculate the # of atoms, particles, grams and particles of the amount given.
where i shows u how the calculate # of moles.
-Finding number of grams with the given molecule and using the periodic table
-Finding the number of particles with the given molecule
Here is a link that might help with the concept of Mole: http://misterguch.brinkster.net/molecalculations.html
Friday, November 19, 2010
November 19 --Even Harder Mole Conversions ~ !
Wednesday, November 17, 2010
Nov 17 2010 - The Mole
Today, we learned about the mole.
The Mole
A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023).
Avogadro's hypothesis
Two equal volumes of gas, at the same temperature and pressure, contain the same number of molecules.
Atomic Mass
The atomic mass (ma) is the mass of a specific isotope of a given atom, most often expressed in unified atomic mass units.
Mass of 1 atom of the element in atomic mass units are called amu or u or daltons.

Lithium has atomic mass of 6.9 amu.
Formula Mass
All the atoms of a formula.

Avogadro's number
The number of particles in a mole of a substance, approximately 6.022x 10^23.
The Mole
A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023).
Avogadro's hypothesis
Two equal volumes of gas, at the same temperature and pressure, contain the same number of molecules.
Atomic Mass
The atomic mass (ma) is the mass of a specific isotope of a given atom, most often expressed in unified atomic mass units.
Mass of 1 atom of the element in atomic mass units are called amu or u or daltons.

Lithium has atomic mass of 6.9 amu.
Formula Mass
All the atoms of a formula.

Avogadro's number
The number of particles in a mole of a substance, approximately 6.022x 10^23.
Sunday, November 14, 2010
November 14 2010 - Review and Density
Today we worked on a review sheet for half the class, which was to help us for the upcoming test. It included scientific notation, significant figures, uncertainty and measurements.
After that we went to the Lab and worked on Excel to plot graphs with data for density. One of our graphs looked somewhat like this.


After that we went to the Lab and worked on Excel to plot graphs with data for density. One of our graphs looked somewhat like this.

Friday, November 5, 2010
November 5, 2010
Today we finally started on our Lab 2E
-Determining the thickness of Aluminum Foil
-We calculated the thickness of a sheet of aluminum foil and expressed the answer with the correct significant figures and scientific notation
-We used 3 pieces of different dimensions of aluminum foil
-Measured the density, the length and width
-From the information and using the formula V=M/D we were able to identify the thickness of the pieces of aluminum foil
Volume song!:
-We learned how to calculate experimental error in %:
(Your answer - Accepted answer)/Accepted answer x 100
Here is a link to a page where it describes this in more detail
REMINDER! next class there is a quiz on this lab so STUDY!
Here is a extra exercise on the Lab we did today:
Thursday, November 4, 2010
November 1 2010 -- Density
Today, we learned about density. Density is mass/volume.
If the density of the object is higher than the density of the liquid it's placed in, the object will sink.
If the density of the object is lower than the density of the liquid it's placed in, the object will float.
A density quiz to keep your mind fresh :) :
http://www.syvum.com/cgi/online/tgamem.cgi/squizzes/physics/density1.tdf?0
If the density of the object is higher than the density of the liquid it's placed in, the object will sink.
If the density of the object is lower than the density of the liquid it's placed in, the object will float.
A density quiz to keep your mind fresh :) :
http://www.syvum.com/cgi/online/tgamem.cgi/squizzes/physics/density1.tdf?0
Sunday, October 31, 2010
October 28 2010 - Uncertainty in Measured Numbers
Today we went over uncertainty in measured numbers.
We can't have perfect measurement. Measurements have uncertainty due to the limits of instruments.
Bob weighs himself on his bathroom scale. The smallest divisions on the scale are 1-pound marks, so the least count of the instrument is 1 pound.
Bob reads his weight as closest to the 142-pound mark. He knows his weight must be larger than 141.5 pounds (or else it would be closer to the 141-pound mark), but smaller than 142.5 pounds (or else it would be closer to the 143-pound mark). So Bob's weight must be
-----------------------------------0.5 pounds
Precision refers to how close together a group of measurements actually are to each other.
Accuracy refers to how close one comes to an accepted value.
We can't have perfect measurement. Measurements have uncertainty due to the limits of instruments.
For example,
Bob weighs himself on his bathroom scale. The smallest divisions on the scale are 1-pound marks, so the least count of the instrument is 1 pound.
Bob reads his weight as closest to the 142-pound mark. He knows his weight must be larger than 141.5 pounds (or else it would be closer to the 141-pound mark), but smaller than 142.5 pounds (or else it would be closer to the 143-pound mark). So Bob's weight must be
weight = 142 +/- 0.5 pounds
---------------------------------------------------------------------------------------------
---------------------------------------------------------------------------------------------
In general, the uncertainty in a single measurement from a single instrument is half the least count of the instrument.
--------------------------------uncertainty in weight
percentage uncertainty = --------------------------- * 100%
---------------------------------value for weight
-----------------------------------0.5 pounds
-------------------------------= --------------- * 100% = 0.35%
----------------------------------142 pounds
Precision refers to how close together a group of measurements actually are to each other.
Accuracy refers to how close one comes to an accepted value.
Ordinarily the more precise measurement if the more accurate.
Thursday, October 28, 2010
Tuesday, October 26, 2010
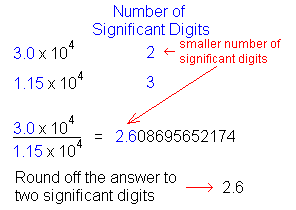
October 26 2010 -- Significant Digits
Today we reviewed the significant digits (or significant figures).
A significant digit is any digit that contributes to giving precision for a number.
Non-zero digits are always significant, however, zeroes follow rules based on their position within a number :
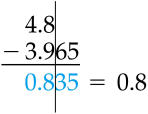
A significant digit is any digit that contributes to giving precision for a number.
Non-zero digits are always significant, however, zeroes follow rules based on their position within a number :
- Zeroes placed before other digits are not significant; 0.046 has two significant digits.
- Zeroes placed between other digits are always significant; 4009 kg has four significant digits.
- Zeroes placed after other digits but behind a decimal point are significant; 7.90 has three significant digits.
- Zeroes at the end of a number are significant only if they are behind a decimal point as in (c). Otherwise, it is impossible to tell if they are significant.
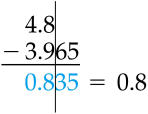
Wednesday, October 20, 2010
October 19 2010 - Lab 3B
-Where we use 3 color dyes (unknown, green and either red, blue, and yellow)
-We used chromatography paper as the object to place the dyes onto and see the filtration of colors happen
-Our color dyes as the solute in this experiment
-The solvent used was water
-As time passed we watched our different dyes, yellow, green and unknown creep up the chromatography paper
-As the water is absorbed and reaches higher and higer, we can see the colors filter out into the primary colors and the dye stretch out and up
-We later understood that unknown dye is a mixture of all the 3 primary colors
-The yellow just filtered out to more yellow
-The unknown dye later filtered out to red, yellow, and blue
-While the green dye filtered out to yellow and blue dye

Here is another video, very similar to the experiment we did :
Monday, October 18, 2010
October 15 2010 -- Separating Mixtures
Separating shows the different components and different properties of the mixtures. You need to think of a process that can separate between components with different properties.
http://sciencepark.etacude.com/projects/separations/separation1.php
This website lists and explains several basic techniques for separation of mixtures, such as filtration, evaporation, crystallization, distillation, and chromatography.
http://sciencepark.etacude.com/projects/separations/separation1.php
This website lists and explains several basic techniques for separation of mixtures, such as filtration, evaporation, crystallization, distillation, and chromatography.
Thursday, October 14, 2010
October 13 2010 - Naming Acids
Monday, October 11, 2010
October 7 2010 -- Ionic and Covalent Compounds
Today we reviewed the properties of ionic and covalent compounds.

Ionic compounds are compounds that are made of 2 or more oppositely charged particles. They are held together by electrostatic forces and electrons are transferred from metal elements to non-metal elements.
In covalent compounds, the electrons are shared between two non-metal elements.

Ionic compounds are compounds that are made of 2 or more oppositely charged particles. They are held together by electrostatic forces and electrons are transferred from metal elements to non-metal elements.
In covalent compounds, the electrons are shared between two non-metal elements.
Thursday, October 7, 2010
October 5 2010 - Lab

-We investigated the heating an
d cooling process for solid and liquid dodecanoic acid. So we'll be able to determine or compare the freezing and melting point.
-We heated and cooled the dodecanoic acid with a hot plate, beaker with water and tube clamps

-When the acid was freezing you can see the white crystals forming and it texture
-When the acid heats up, it is very noticeable, like melting ice but the acid is yellowish white
-We used a thermometer to measure the temperature s of the freezing and melting points of the acid
-We determined that the freezing point was 37'C and melting was 47'C
Monday, October 4, 2010
October 1 2010 -- Matter Is Made of Atoms
Atoms
• Atom – smallest possible piece of something

Elements
• Elements – pure substances that cannot be broken down
• Contains only one kind of atom
• Can exist as a solid, liquid, or gas
• Molecules – particles made of more than one atom
• Have different melting points and boiling points because they vary in mass and size
o The larger the particle, the higher the boiling point
Compounds
• Made by combining elements in definite properties
• Can also exist as solids, liquids, and gases
• Ions – particles that have an electrical charge

• Atom – smallest possible piece of something

Elements
• Elements – pure substances that cannot be broken down
• Contains only one kind of atom
• Can exist as a solid, liquid, or gas
• Molecules – particles made of more than one atom
• Have different melting points and boiling points because they vary in mass and size
o The larger the particle, the higher the boiling point
Compounds
• Made by combining elements in definite properties
• Can also exist as solids, liquids, and gases
• Ions – particles that have an electrical charge

October 1 2010 -- Matter in the Macroscopic World (text)
What You Know About Matter
• Water normally exists as a liquid that pours freely to fill any solid container, and will solidify if cooled, or evaporate if heated
• Properties such as color and taste of characteristics of matter
Purifying Matter
• Mixture – two (or more) kinds of matter that have separate identities
o Easily separated into component parts
o Said to be impure
o Mixtures like salt water or sugar water that look uniform throughout and do not scatter light are called solutions
o Separating solutions like salt water or sugar water by boiling them dry on the stove is called distillation
Characteristics of Pure Substances
• Pure substances have a constant boiling point
• Freezing point – the temperature at which a liquid solidifies
• Melting point – the temperature at which a solid liquefies
Chemical and Physical Changes
• Density – a property of matter that describes its mass per unit volume
• Chemical change – changes that produce a new kind of matter with different properties
• Decomposition – when one kind of matter breaks apart to create two or more kinds of matter
• Physical change – changes that are easily reversed to get the original material back again; do not appear to produce new kinds of matter
Compounds and Elements
• Electrolysis – involves passing an electric current through a substance to make it decompose into new kinds of matter
• Compounds – pure substances that can be decomposed into new kinds of matter
• Elements – elemental building blocks of all kinds of matter; cannot be decomposed
o 109 known elements

• Water normally exists as a liquid that pours freely to fill any solid container, and will solidify if cooled, or evaporate if heated
• Properties such as color and taste of characteristics of matter
Purifying Matter
• Mixture – two (or more) kinds of matter that have separate identities
o Easily separated into component parts
o Said to be impure
o Mixtures like salt water or sugar water that look uniform throughout and do not scatter light are called solutions
o Separating solutions like salt water or sugar water by boiling them dry on the stove is called distillation
Characteristics of Pure Substances

• Pure substances have a constant boiling point
• Freezing point – the temperature at which a liquid solidifies
• Melting point – the temperature at which a solid liquefies
Chemical and Physical Changes
• Density – a property of matter that describes its mass per unit volume
• Chemical change – changes that produce a new kind of matter with different properties
• Decomposition – when one kind of matter breaks apart to create two or more kinds of matter
• Physical change – changes that are easily reversed to get the original material back again; do not appear to produce new kinds of matter
Compounds and Elements
• Electrolysis – involves passing an electric current through a substance to make it decompose into new kinds of matter
• Compounds – pure substances that can be decomposed into new kinds of matter
• Elements – elemental building blocks of all kinds of matter; cannot be decomposed
o 109 known elements

Sunday, October 3, 2010
October 1 2010 -- New Laws and the Heating/Cooling Curve of a Pure Substance (notes)
On Friday, we started a new topic about matter and the heating/cooling curve of a pure substance.
Law of Definite Composition
Compounds will have a definite composition. For example, water (H2O) will be water anywhere (it always have 2 hydrogens and 1 oxygen).
Law of Multiple Proportions – when two or more compounds with different properties of the same elements can be made. For example, carbon dioxide (CO2) x 2 = dicarbon tetraoxide/oxalate (C2O4).

A : At this point, the pure substance is in the solid state, because the pure substance is below the melting point. The particles are packed together closely in an orderly manner. Particles can only vibrate at a fixed position and the forces between the particles are very strong.
A-B : When the pure substance is heated, the heat energy is converted to kinetic energy. The molecules vibrate faster in their fixed positions and the temperature increases when the kinetic energy increases.
B : The pure substance is still solid, but the melting stage has begun – the solid begins to change into a liquid. The temperature remains the same.
B-C : The pure substance exists in both solid and liquid states. The heat that is supplied to it is used to overcome the forces of attraction that holds the particles together because the temperature remains constant. This constant temperature is called the melting point. The latent heat of fusion is the heat energy absorbed to overcome the intermolecular forces.
C : The pure substance has completely melted, and the solid has turned to liquid.
C-D : The pure substance is in liquid state, and as the liquid is heated and the temperature is increased, the particles move faster because the kinetic energy is increasing.
D : The pure substance is still in liquid state, and the molecules have gained enough kinetic energy to overcome the forces of attraction between the particles. Some of the molecules begin to change into gas.
D-E : The pure substance is in both liquid and gaseous states. The temperature is unchanged, and the heat energy absorbed is used to overcome the molecular forces between the particles of the liquid rather than increase the temperature. This constant temperature is called the boiling point.
E : All of the pure substance liquid has turned into gas.
E-F : The gas particles continue to absorb more energy and move faster. The temperature increases as heating continues.
Law of Definite Composition
Compounds will have a definite composition. For example, water (H2O) will be water anywhere (it always have 2 hydrogens and 1 oxygen).
Law of Multiple Proportions – when two or more compounds with different properties of the same elements can be made. For example, carbon dioxide (CO2) x 2 = dicarbon tetraoxide/oxalate (C2O4).

A : At this point, the pure substance is in the solid state, because the pure substance is below the melting point. The particles are packed together closely in an orderly manner. Particles can only vibrate at a fixed position and the forces between the particles are very strong.
A-B : When the pure substance is heated, the heat energy is converted to kinetic energy. The molecules vibrate faster in their fixed positions and the temperature increases when the kinetic energy increases.
B : The pure substance is still solid, but the melting stage has begun – the solid begins to change into a liquid. The temperature remains the same.
B-C : The pure substance exists in both solid and liquid states. The heat that is supplied to it is used to overcome the forces of attraction that holds the particles together because the temperature remains constant. This constant temperature is called the melting point. The latent heat of fusion is the heat energy absorbed to overcome the intermolecular forces.
C : The pure substance has completely melted, and the solid has turned to liquid.
C-D : The pure substance is in liquid state, and as the liquid is heated and the temperature is increased, the particles move faster because the kinetic energy is increasing.
D : The pure substance is still in liquid state, and the molecules have gained enough kinetic energy to overcome the forces of attraction between the particles. Some of the molecules begin to change into gas.
D-E : The pure substance is in both liquid and gaseous states. The temperature is unchanged, and the heat energy absorbed is used to overcome the molecular forces between the particles of the liquid rather than increase the temperature. This constant temperature is called the boiling point.
E : All of the pure substance liquid has turned into gas.
E-F : The gas particles continue to absorb more energy and move faster. The temperature increases as heating continues.
Thursday, September 30, 2010
September 29 2010 - Lab 2C Chemical and Physical Change
Today, we did a lab for chemiacal and physical change.
Purpose of this lab is to find out characteristic of chemical and physical changes and to know difference between chemical change and physical change.
Material :
Equipment
4 small test tubes glass square
(10mm x 75mm) lab apron
test-tube rack safety goggles
4 medicine droppers
Chemical Reagents
set of 4 unknown solutions
Procedure :

Experimental Results :

As a result, combination of solution D and A, D and B, and D and C are chemically changed.
These combinations have characteristics of chemical changes that changed color. Also combination of solution B and C is a chemical change due to characteristic of chemical change which is forming new substances.
Purpose of this lab is to find out characteristic of chemical and physical changes and to know difference between chemical change and physical change.
Material :
Equipment
4 small test tubes glass square
(10mm x 75mm) lab apron
test-tube rack safety goggles
4 medicine droppers
Chemical Reagents
set of 4 unknown solutions
Procedure :

Experimental Results :

As a result, combination of solution D and A, D and B, and D and C are chemically changed.
These combinations have characteristics of chemical changes that changed color. Also combination of solution B and C is a chemical change due to characteristic of chemical change which is forming new substances.
Tuesday, September 28, 2010
September 27 2010 - Matter and Physical/Chemical Change
Today, we went over a common topic in science: matter. Matter is any object that takes up space and has a mass.
Matter is split into two categories: pure substances and mixtures.

Pure substances have only one set of properties, and only one kind of particle. Pure substances are split into elements, which are the simplest form and are made out of atoms (metal, non-metal and metalloid), and compounds, which are chemically compounded, made out of elements, and have small particles within them called molecules, which can be ionic or covalent.
Mixtures are the complete opposite, having more than one set of properties. Mixtures are grouped into homogeneous, being uniform throughout and only having one component, and heterogeneous, being non-uniform and having more than one component.
We also reviewed physical and chemical change, mainly looking at their differences.

Physical change is reversible, while chemical change isn't. It doesn't add any new substances into the product, unlike chemical change. Physical change also leaves the chemical composition the same as it was before the change.
Matter is split into two categories: pure substances and mixtures.

Pure substances have only one set of properties, and only one kind of particle. Pure substances are split into elements, which are the simplest form and are made out of atoms (metal, non-metal and metalloid), and compounds, which are chemically compounded, made out of elements, and have small particles within them called molecules, which can be ionic or covalent.
Mixtures are the complete opposite, having more than one set of properties. Mixtures are grouped into homogeneous, being uniform throughout and only having one component, and heterogeneous, being non-uniform and having more than one component.
We also reviewed physical and chemical change, mainly looking at their differences.

Physical change is reversible, while chemical change isn't. It doesn't add any new substances into the product, unlike chemical change. Physical change also leaves the chemical composition the same as it was before the change.
Saturday, September 25, 2010
September 23 2010 - Review
Today, we review unit conversion and unitary rates. Which we have done many reviews and worksheets for.
Not only did we review but we also had a Practice Quiz on the material we will be quized or tested on next week. Which includes :
-Unit Conversions- ( giv_-_Metric_Conversions.gif) en quantities and derived unites)
en quantities and derived unites)
_-_Metric_Conversions.gif) en quantities and derived unites)
en quantities and derived unites)eg. Convert 3 s into milliseconds
eg. Convert 28.4 g/mL into kilograms per litre
-Unitary Rates-
eg. Convert mm into km

-Scientific Notation
eg. Put 1232133 km into scientific notation
(1.232133 x 107)
Also i really enjoyed the shorter 50 minute class ;D
Wednesday, September 22, 2010
September 21 2010 - Unitary Rates
We re-reviewed Unitary Rates, which we've gone over many times.
Quantities are combinations of numbers and units. You have to have a combination of numbers and units because they don't make sense without each other. It'd be like saying, "This costs 457.09" or "I ate 9" (assuming, of course, that you aren't 7. HARHARHHAR MATH JOKE)
The Systeme Internationale, or the SI, is a French system using powers of 10. We call this system the Scientific Notation system (covered in the last post).
A handy unit conversion chart :
http://www.austinpowder.com/BlastersGuide/blasterstools/_graphics/table-unit-conversion.gif
- Esther
Quantities are combinations of numbers and units. You have to have a combination of numbers and units because they don't make sense without each other. It'd be like saying, "This costs 457.09" or "I ate 9" (assuming, of course, that you aren't 7. HARHARHHAR MATH JOKE)
The Systeme Internationale, or the SI, is a French system using powers of 10. We call this system the Scientific Notation system (covered in the last post).
A handy unit conversion chart :
http://www.austinpowder.com/BlastersGuide/blasterstools/_graphics/table-unit-conversion.gif
- Esther
Tuesday, September 21, 2010
September 21 2010 - Scientific Notation
First blog post! :) (other than the test post. That doesn't count)
Today, in class, we learned about Scientific Notation. We should have already learned this in grade 9 already, so this was more of a review.
Scientific notation is used to express very large or very small numbers using powers of 10.
For example, 25,100,000,000 would be expressed as 2.51x10^10, because for scientific notation, the number in the front has to be a number bigger than 1 and less than 10 (eg. 2.5, 6.7, 2.78). First, you move the decimal point (which is after the last digit [eg. 3487000 would be 3487000.0]) to the left until the number is between 0 and 10. Then, count the number of decimal places you moved over, and beside the new number, add a 10^# (10 to the power of #).
0.000000030 would be expressed as 3.02x10^-8. For negative equations, you move the decimal to the right instead of the left.

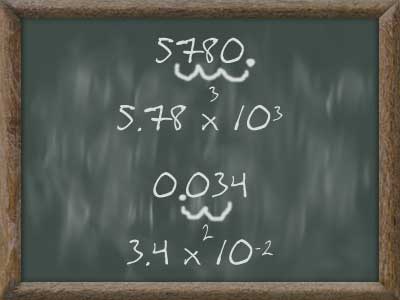
On the other hand, 3.25x10^8 would be expressed as 325,000,000, because you would move the decimal place over 8 spaces.

How to use Scientific Notation on a calculator:
1. Punch in the decimal number from 1-10
2. Press the 'EE' button
3. Punch in the power (10^17, press in 17, NOT 10^17)
4. Press ENTER :)
Alternate way (for dummies)
1. Punch in the decimal number from 1-10
2. Punch in 10
3. Punch in the (^) symbol
4. Punch in the power
5. Press enter :D
- Esther
Today, in class, we learned about Scientific Notation. We should have already learned this in grade 9 already, so this was more of a review.
Scientific notation is used to express very large or very small numbers using powers of 10.
For example, 25,100,000,000 would be expressed as 2.51x10^10, because for scientific notation, the number in the front has to be a number bigger than 1 and less than 10 (eg. 2.5, 6.7, 2.78). First, you move the decimal point (which is after the last digit [eg. 3487000 would be 3487000.0]) to the left until the number is between 0 and 10. Then, count the number of decimal places you moved over, and beside the new number, add a 10^# (10 to the power of #).
0.000000030 would be expressed as 3.02x10^-8. For negative equations, you move the decimal to the right instead of the left.

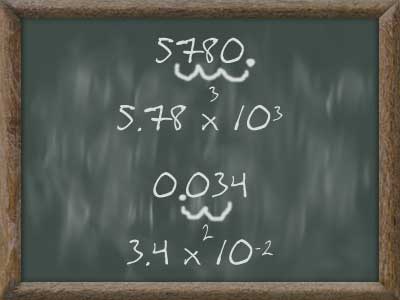
On the other hand, 3.25x10^8 would be expressed as 325,000,000, because you would move the decimal place over 8 spaces.

How to use Scientific Notation on a calculator:
1. Punch in the decimal number from 1-10
2. Press the 'EE' button
3. Punch in the power (10^17, press in 17, NOT 10^17)
4. Press ENTER :)
Alternate way (for dummies)
1. Punch in the decimal number from 1-10
2. Punch in 10
3. Punch in the (^) symbol
4. Punch in the power
5. Press enter :D
- Esther
Monday, September 20, 2010
Subscribe to:
Posts (Atom)


