Today we went over percent composition.
Percent composition of a compound shows that how much of each elements in the compound and which elements are in the compound.
Percentage compound = mass of element / mass of compound x 100 %
Ex 1. What is the percentage composition of CuCl2?
Total MM(Molar Mass) = 134.5g/mol
% of Cu = 63.5/134.5 x 100 % = 47.2 %
% of Cl = 71.0/134.5 x 100% = 52.8 %
2. What is the percentage ocomposition of CaO?
Total MM = 56.1g/mol
% of Ca = 40.1/56.1 x 100% = 71.5 %
% of O = 16.0/56.1 x 100 % = 29.5
3. Calculate percentage composition of the bolded components of Na2SO4.
Total MM = 142.1g/mol
MM of all the SO4 = 96.1g/mol
% of SO4 = 96.1/142.1 x 100 % = 67.6 %
4. Calculate percentage composition of NaCl.
Total MM = 58.5g/mol
% of Na = 23.0/58.5 x 100 % = 39.3 %
% of Cl = 35.5/58.5 x 100% = 60.7 %
5. Calculate the percentage of bolded components of (NH4)3PO4.
Total MM = 149.1
MM of all the NH4 = 54.0
% of NH4 = 54.0 / 149.1 x 100 % = 36.2 %
Tuesday, November 30, 2010
Tuesday, November 23, 2010
November 23, 2010
Today, we went more in detail the amazing wonders of Mole, and the conversion powers it possess.
-Not only are we able to calculate the number of particles, formula units, molecules, atoms from Avogadro's number
-We are now able to use the molar mass of any molecule to calculate the # of atoms, particles, grams and particles of the amount given.
where i shows u how the calculate # of moles.
-Finding number of grams with the given molecule and using the periodic table
-Finding the number of particles with the given molecule
Here is a link that might help with the concept of Mole: http://misterguch.brinkster.net/molecalculations.html
Friday, November 19, 2010
November 19 --Even Harder Mole Conversions ~ !
Wednesday, November 17, 2010
Nov 17 2010 - The Mole
Today, we learned about the mole.
The Mole
A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023).
Avogadro's hypothesis
Two equal volumes of gas, at the same temperature and pressure, contain the same number of molecules.
Atomic Mass
The atomic mass (ma) is the mass of a specific isotope of a given atom, most often expressed in unified atomic mass units.
Mass of 1 atom of the element in atomic mass units are called amu or u or daltons.

Lithium has atomic mass of 6.9 amu.
Formula Mass
All the atoms of a formula.

Avogadro's number
The number of particles in a mole of a substance, approximately 6.022x 10^23.
The Mole
A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023).
Avogadro's hypothesis
Two equal volumes of gas, at the same temperature and pressure, contain the same number of molecules.
Atomic Mass
The atomic mass (ma) is the mass of a specific isotope of a given atom, most often expressed in unified atomic mass units.
Mass of 1 atom of the element in atomic mass units are called amu or u or daltons.

Lithium has atomic mass of 6.9 amu.
Formula Mass
All the atoms of a formula.

Avogadro's number
The number of particles in a mole of a substance, approximately 6.022x 10^23.
Sunday, November 14, 2010
November 14 2010 - Review and Density
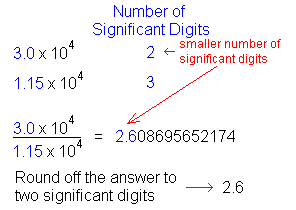
Today we worked on a review sheet for half the class, which was to help us for the upcoming test. It included scientific notation, significant figures, uncertainty and measurements.
After that we went to the Lab and worked on Excel to plot graphs with data for density. One of our graphs looked somewhat like this.


After that we went to the Lab and worked on Excel to plot graphs with data for density. One of our graphs looked somewhat like this.

Friday, November 5, 2010
November 5, 2010
Today we finally started on our Lab 2E
-Determining the thickness of Aluminum Foil
-We calculated the thickness of a sheet of aluminum foil and expressed the answer with the correct significant figures and scientific notation
-We used 3 pieces of different dimensions of aluminum foil
-Measured the density, the length and width
-From the information and using the formula V=M/D we were able to identify the thickness of the pieces of aluminum foil
Volume song!:
-We learned how to calculate experimental error in %:
(Your answer - Accepted answer)/Accepted answer x 100
Here is a link to a page where it describes this in more detail
REMINDER! next class there is a quiz on this lab so STUDY!
Here is a extra exercise on the Lab we did today:
Thursday, November 4, 2010
November 1 2010 -- Density
Today, we learned about density. Density is mass/volume.
If the density of the object is higher than the density of the liquid it's placed in, the object will sink.
If the density of the object is lower than the density of the liquid it's placed in, the object will float.
A density quiz to keep your mind fresh :) :
http://www.syvum.com/cgi/online/tgamem.cgi/squizzes/physics/density1.tdf?0
If the density of the object is higher than the density of the liquid it's placed in, the object will sink.
If the density of the object is lower than the density of the liquid it's placed in, the object will float.
A density quiz to keep your mind fresh :) :
http://www.syvum.com/cgi/online/tgamem.cgi/squizzes/physics/density1.tdf?0
Subscribe to:
Posts (Atom)
