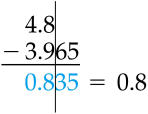Today we went over uncertainty in measured numbers.
We can't have perfect measurement. Measurements have uncertainty due to the limits of instruments.
Bob weighs himself on his bathroom scale. The smallest divisions on the scale are 1-pound marks, so the least count of the instrument is 1 pound.
Bob reads his weight as closest to the 142-pound mark. He knows his weight must be larger than 141.5 pounds (or else it would be closer to the 141-pound mark), but smaller than 142.5 pounds (or else it would be closer to the 143-pound mark). So Bob's weight must be
-----------------------------------0.5 pounds
Precision refers to how close together a group of measurements actually are to each other.
Accuracy refers to how close one comes to an accepted value.
We can't have perfect measurement. Measurements have uncertainty due to the limits of instruments.
For example,
Bob weighs himself on his bathroom scale. The smallest divisions on the scale are 1-pound marks, so the least count of the instrument is 1 pound.
Bob reads his weight as closest to the 142-pound mark. He knows his weight must be larger than 141.5 pounds (or else it would be closer to the 141-pound mark), but smaller than 142.5 pounds (or else it would be closer to the 143-pound mark). So Bob's weight must be
weight = 142 +/- 0.5 pounds
---------------------------------------------------------------------------------------------
---------------------------------------------------------------------------------------------
In general, the uncertainty in a single measurement from a single instrument is half the least count of the instrument.
--------------------------------uncertainty in weight
percentage uncertainty = --------------------------- * 100%
---------------------------------value for weight
-----------------------------------0.5 pounds
-------------------------------= --------------- * 100% = 0.35%
----------------------------------142 pounds
Precision refers to how close together a group of measurements actually are to each other.
Accuracy refers to how close one comes to an accepted value.
Ordinarily the more precise measurement if the more accurate.