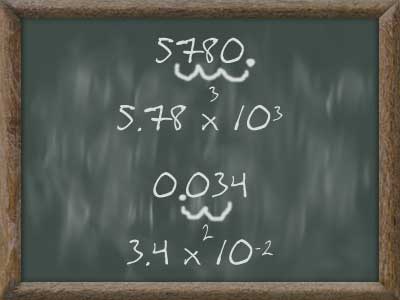Today, we did a lab for chemiacal and physical change.
Purpose of this lab is to find out characteristic of chemical and physical changes and to know difference between chemical change and physical change.
Material :
Equipment
4 small test tubes glass square
(10mm x 75mm) lab apron
test-tube rack safety goggles
4 medicine droppers
Chemical Reagents
set of 4 unknown solutions
Procedure :

Experimental Results :

As a result, combination of solution D and A, D and B, and D and C are chemically changed.
These combinations have characteristics of chemical changes that changed color. Also combination of solution B and C is a chemical change due to characteristic of chemical change which is forming new substances.
Purpose of this lab is to find out characteristic of chemical and physical changes and to know difference between chemical change and physical change.
Material :
Equipment
4 small test tubes glass square
(10mm x 75mm) lab apron
test-tube rack safety goggles
4 medicine droppers
Chemical Reagents
set of 4 unknown solutions
Procedure :

Experimental Results :

As a result, combination of solution D and A, D and B, and D and C are chemically changed.
These combinations have characteristics of chemical changes that changed color. Also combination of solution B and C is a chemical change due to characteristic of chemical change which is forming new substances.


_-_Metric_Conversions.gif) en quantities and derived unites)
en quantities and derived unites)